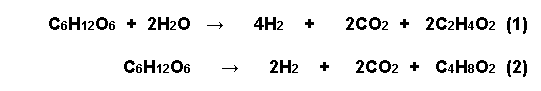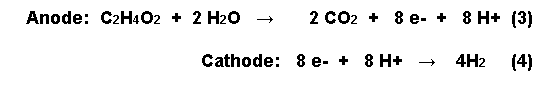The
Institute of Science in Society
ISIS Press Release 03/06/05
Bug Power
Waste-gobbling
bacteria may be our dream ticket to clean
renewable energy. Dr. Mae-Wan Ho
A fully referenced version of this paper is posted on ISIS
members’ website. Details here
Resources and energy
from wastes
Bacteria that gobble
wastes are a godsend. They prevent the build up
of wastes in our environment and play an
indispensable role in making wastewater safe for
domestic animals, wild life, and human beings. In
many Third World countries, these same bacteria
are working miracles turning manure and other
wastes into valuable resources to support highly
productive farms that require no input and
generate little or no waste ("Dream
farm", this series). When these bacteria are
confined in anaerobic digesters with limited or
no access to oxygen, they ferment the wastes,
release and conserve nutrients for livestock and
crops, and produce ‘biogas’ as
by-product, which typically consists of about 60%
methane (CH4) and a small amount of hydrogen
(H2), both of which can be burnt as
smokeless fuel.
Within the past two
years, these same bacteria are showing even more
remarkable potential for producing clean and
renewable energy while reducing greenhouse gas
emissions.
Hydrogen economy on
potato waste
The "hydrogen
economy" is on everyone’s lips as the
answer to the ultimate clean energy. Burning
hydrogen produces pure water instead of green
house gases, and it is by far the most energetic
fuel on earth, weight for weight. But in order to
really reduce green house gas emissions, hydrogen
must be produced sustainably with renewable
sources such as sun, wind and biomass. About half
of all hydrogen produced currently is from
natural gas, the rest is produced primarily using
other fossil fuels. Only 4% is generated by
splitting water using electricity derived from a
variety of sources.
At BIOCAP Canada’s
First National Conference in February 2005, a
research team at the Wastewater Technology Centre
and the University of Waterloo in Ontario,
Canada, presented a poster describing a prototype
process for producing substantial amounts of
hydrogen as well as methane from potato waste
[1].
The team used a
two-stage anaerobic digestion to get first
hydrogen and then methane. In this way, it was
possible to optimize the first stage for
producing hydrogen. The key appears to be an
acidic pH of 5.5 in the hydrogen reactor, instead
of pH 7 in the methane reactor. Both reactors
were run at 35C.
They pulped the potatoes
bought from a store and treated the slurry with
peptone (an enzyme that breaks down protein),
then seeded the two reactors – one for
hydrogen the other for methane - with digested
sludge from the local wastewater treatment plant
to get the bacteria in place. For the hydrogen
reactor, the seed sludge was pre-cultivated in a
sucrose medium for a few days before switching to
potato waste when high hydrogen production was
confirmed. For the methane reaction, no
precultivation of the sludge was required.
From the 4th
day, the potato pulp replaced sucrose and
hydrogen biogas was produced continuously for a
further 90 days. The maximum production rate from
the one litre reactor was 270ml/h on the 17th
day, and the average rate over the entire 90-day
period was 112.2ml/h. The hydrogen fraction
fluctuated between 39 and 51 percent of the
biogas (v/v). The average chemical oxygen demand
(COD) concentration (a measure of the amount of
waste present) of the fluid coming out of the
hydrogen reactor was 7 220mg/L, at an input
concentration of 12 800mg/L. So more than 40
percent of the waste was removed.
Once hydrogen production
became stable after the 20th day, the
outflow from the hydrogen reactor was transferred
to the second, bigger (methane) reactor, 5 litres
in volume. During the 70 days of operation,
methane biogas was produced continuously; the
maximum rate was 410ml/h, and the average rate,
213 ml/h. The concentration of methane in the
biogas was between 69 and 79 percent. The average
COD concentration in the methane bioreactor
outflow was 4 130 mg/L. Again, the process
removed more than 40% of the wastes. Together,
the two reactors removed 68% of the waste.
Based on the hydrogen
and methane production rates, the average energy
yield from each kilogram dry weight of potato
waste was 4.96 MJ (1.4kWh) and the maximum energy
yield, 9.58 MJ (2.7kWh). For comparison, burning
1 kg wood yields about 20MJ [2]. But because the
energy is generated from waste, it is essentially
free, and does not require chopping down trees.
Potato is the third
largest food crop in the world, and Canada is one
of the leading producers (4.7million tonnes
annually). Large amounts of potato waste come
from food and potato processing plants. This is
potentially a huge source of renewable, clean
energy.
Dual purpose microbial
fuel cell
A research team in
Pennsylvania State University has also discovered
how to coax the same bugs to make plenty of
hydrogen while they are gobbling wastes [3].
When the bacteria
ferment glucose, they generate a maximum of 4
molecules of hydrogen per molecule of glucose and
end up at best with two molecules of acetic acid
that they cannot convert further to hydrogen due
to an electrochemical barrier. But, given a
little electrical boost, the bacteria can jump
over the barrier to generate more hydrogen.
The research team, led
by Dr. Bruce Logan, already made news in 2004
[4], when they succeeded in getting the bacteria
to produce electricity while removing wastes.
The bacteria were put
into a microbial fuel cell that generated 26mW m2 of electricity while removing up
to 80% of the wastes that flowed through.
These waste treatment
bacteria, numerous species belonging to many
genera including Geobacter, Shewanella,
and Pseudomonas, have the ability to
transfer electrons obtained by fermenting wastes
to external metals [5]. When the bacteria are
attached to electrodes, the electrons are
transferred to the electrodes (the anode), to
flow through an external circuit to the cathode
where they combine with oxygen from the air and
protons (hydrogen ions) to form water.
The reactor then used
was a single cylindrical plexiglass chamber the
size of a soda water bottle in which the anode,
consisting of eight graphite rods, was placed in
a concentric arrangement surrounding a central
cathode that was exposed to air. The air-porous
cathode consisted of a carbon/platinum
catalyst/proton exchange membrane layer fused to
a plastic support tube.
The efficiency of the
system, based on waste removal and current
generation was less than 12%, indicating that a
substantial fraction of the organic matter was
lost without generating current; perhaps in
producing more bacteria. But as the bacteria were
doing their intended job, which was to remove
waste, any electricity generated at the same time
was an extra bonus.
Excluding air and
boosting electric potential
Now, the team has
discovered that by excluding air from the
cathode, and by giving the bugs a boost of about
250mV, they can make the bugs produce hydrogen at
high efficiency. They refer to this process as
electrochemically assisted microbial production
of hydrogen.
Normal fermentation
converts glucose to dead end products such as
acetic and butyric acid:
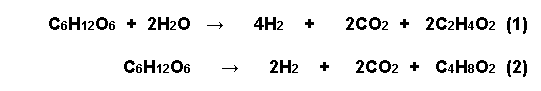
In the first case, four
molecules of hydrogen are generated, and in the
second, only two molecules. The greatest
theoretical yield possible is four molecules of
hydrogen per molecule of glucose.
The microbial fuel cell,
however, offers a new solution to the problem. By
augmenting the electric potential in the
microbial fuel cell circuit, it gave just the
little help needed for the bacteria to make
hydrogen out of acetic acid.
In a typical fuel cell,
the open circuit potential of the anode is about
–300mV. If hydrogen is produced at the
cathode, the half reactions occurring at the
anode and the cathode with acetic acid oxidized
at the anode, are as follows:
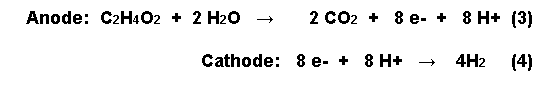
In order for the bugs to
donate electrons to the anode from acetic acid,
however, the anode potential has to be made less
electronegative.
To improve the
efficiency of the intended process, the
researchers also created a two chamber microbial
fuel cell instead of the one-chamber version they
had previously constructed. One chamber contained
the anode, the other the cathode, separated by a
proton exchange membrane. A major advantage of
housing anode and cathode in separate chambers is
that the hydrogen produced at the cathode is
separated from the carbon dioxide at the anode at
source. Instead of being exposed to air, the
cathode chamber was sealed. A voltage of 250mV or
greater was applied to the circuit by connecting
the positive pole of a power supply to the anode,
and the negative pole to the cathode.
The external power
supply increased the anode potential from –300mV
to -291mV with a boost of 250mV and to –275mV
with a boost of 850mV, producing hydrogen and
degrading more than 95% of the acetate in the
process. The recovery of electrons as hydrogen
was over 90%. The Coulombic efficiency - defined
as the recovery of total electrons in acetate as
current - ranged from 60 to 78% depending on the
applied voltage. Thus 2.9 of the theoretical
maximum 4 molecules of hydrogen are obtained from
the acetic acid reaction with water by an
injection of 250mV of electricity (see equation
3). This compares favourably with the
costly1800-2000 mV needed for getting hydrogen
from splitting water [6].
A combined fermentation
and bioelectrochemically assisted anaerobic
microbial fuel cell has the potential to produce
as much as 8 to 9 molecules of hydrogen starting
from a molecule of glucose (The theoretical
maximum is 12, see equations 1, 3 and 4.)
With this
bioelectrochemically-assisted reactor, hydrogen
can be produced from any type of biodegradable
organic matter. Combined hydrogen production and
wastewater treatment will offset the substantial
costs of wastewater treatment as well as provide
a contribution to the hydrogen economy. As the
technology is rather simple, it can be adapted
for use at different scales, in third world
countries as well as industrialised countries.
At the BIOCAP Canada
conference referred to earlier, another poster
pointed out that 45 of 56 wastewater treatment
plants in large urban areas of Ontario, Canada
incorporate an anaerobic digestion process to
reduce the volume of disposable sludge; but the
methane produced is mostly wasted by being flared
off to the atmosphere. A conservative estimate
suggests that if all the wastewater sites were to
use anaerobic digesters and simply recover the
methane to generate electricity, this would
produce 1.51 GWh/day [7]. It was a small
percentage of the total of 317GWh consumed each
day in Ontario. But on average, 0.3kg of CO2 is emitted per kWh energy
produced from Ontario Power Generation, so simply
recovering the biogas energy from the current
sites using anaerobic digesters represents a
saving of 432 tonnes of CO2 per day.
Imagine what can be
achieved if waste treatment were optimised for
hydrogen production.
Support our
Sustainable World Global Initiative and sign up
for the First International Conference now http://www.i-
sis.org.uk/SWCFA.php
|